Curevac Vaccine Covid
The jab developed with GSK has shown better results in early-phase animal trials and appears to elicit 10 times more antibodies than the first vaccine. CureVac stock fell after the company decided to stop developing an mRNA vaccine for Covid-19.

Germany S Bayer To Cooperate With Curevac On Vaccine
CureVacs Covid-19 vaccine is now the seventh to be abandoned after entering clinical trials.

Curevac vaccine covid. CVAC has decided to withdraw the companys first-generation COVID-19 vaccine candidate CVnCoV from the current. CureVac fell far behind rivals BioNTech a partner of Pfizer and Moderna in trying to develop an mRNA-based COVID-19 vaccine. The global fight against Covid-19 continues and we remain committed to.
The vaccine will be an important contribution to help manage the Covid-19 pandemic and the dynamic variant spread Franz-Werner Haas the chief executive of CureVac said in the announcement. CureVac will withdraw its first-generation COVID-19 vaccine candidate and focus on collaborating with GSK to develop second-generation mRNA vaccine technology instead the Germany-based biotechnology company said on Tuesday. Last month Sanofi announced it was giving up on its mRNA vaccine.
CureVacs experimental COVID-19 vaccine failed in a pivotal clinical study the biotech said Wednesday. A volunteer receives a dose of CureVac vaccine or a placebo during a study by the German biotech firm CureVac as part of a testing for a new vaccine against the coronavirus disease COVID-19 in. A dose of CureVac vaccine or a placebo is seen during a study by the German biotech firm CureVac as part of a testing for a new vaccine against the coronavirus disease COVID-19 in Brussels.
Its the first late-stage study to flop with CureVacs vaccine showing 47 effectiveness. This collaboration will build on CureVacs first generation COVID-19 vaccine candidate CVnCoV which is currently in Phase 2b3 clinical trial and on CureVacs ability to optimise mRNA for a strong immune response manufacturability and stability at standard 2. CureVacs COVID-19 Vaccine Candidate CVnCoV Demonstrated Efficient Protection of Non-Human Primates During SARS-CoV-2 Challenge Infection Data provided further evidence on immunogenicity and protective efficacy of CVnCoV.
CureVac was the first to use messenger RNA technology in medicine and thats now been deployed in the Pfizer-BioNTech and Moderna COVID-19 vaccines. Shares of CureVac CVAC -461 tumbled 96 in premarket trading on Tuesday after the German biopharmaceutical company said it will scrap development of. CureVac said it would abandon its application for approval from the European Medicines Agency for its first COVID-19 vaccine candidate CVnCoV after late-stage trials delivered disappointing.
CureVac stock fell 145 in premarket trading. The CureVac-GSK COVID-19 collaboration announced in February 2021 extends the existing strategic mRNA technology partnership both companies started in July 2020 which focuses on the development of new products based on CureVacs second-generation RNA-technology for different targets in the field of infectious diseases. But CureVacs newer version may.
Induction of robust antibody and T cell responses at lower dose than tested in Phase 3 trial. CureVac said early Tuesday it was halting development of its first messenger RNA-based Covid-19 vaccine and would instead focus on. RTTNews - CureVac NV.
CureVacs Second-Generation mRNA COVID-19 Vaccine Candidate Shows Promise. The company said it will withdraw its first-generation vaccine candidate CVnCoV which failed late-stage trials with 47 accuracy in June due to a potential. Germanys CureVac NV said it would shelve its most advanced Covid-19 vaccine and focus on a new version in the latest sign that efforts to develop vaccines against the virus are entering a new.
CureVac in collaboration with London-based GlaxoSmithKline also has a second-generation COVID-19 vaccine in the works that like its predecessor uses unmodified mRNA but has been fine-tuned so. Shares of CureVac tumbled 96 in premarket trading on Tuesday after the German biopharmaceutical company said it will scrap development of its COVID-19 vaccine candidate and instead focus on. Second-generation lead COVID-19 vaccine candidate CV2CoV developed in collaboration by CureVac and GSK CV2CoV mRNA shows high levels of antigen production in rat model.
The BioNTech-Pfizer alliance in particular has been a dominant force among suppliers to Western countries with well over 1 billion doses delivered so far. CureVac and GSK have published preclinical data evaluating the immune responses and protective efficacy of CureVacs first-generation vaccine candidate CVnCoV and the second-generation vaccine candidate CV2CoV against SARS-CoV-2 in non-human primates. Curevacs US-listed shares fell 8 per cent.

Curevac Says Its Covid 19 Vaccine Was 47 Efficacious In Trial Beset By Variants

European Commission Wraps Up Curevac Covid 19 Vaccine Supply Deal

Curevac Initiates Phase Iib Iii Trial Of Covid 19 Vaccine Candidate
Curevac Vaccine Performs At 47 Efficacy In Latest Trial
Curevac Launches Final Trials For Covid 19 Vaccine
Gsk And Curevac S Second Covid Vaccine Yields Stronger Response Financial Times
Germany Says Vaccine Rollout On Track Despite Curevac Trial Flop Reuters

Study Shows Curevac Vaccine Is 100 Effective Against Death From Covid Tecnologico De Monterrey

Curevac S Road To The Coronavirus Vaccine Juve Patent

Curevac Q A Resolving Cold Chain Logstics Of Covid 19 Mrna Vaccines

Pride And Hope The Curevac Story Berlin Policy Journal Blog
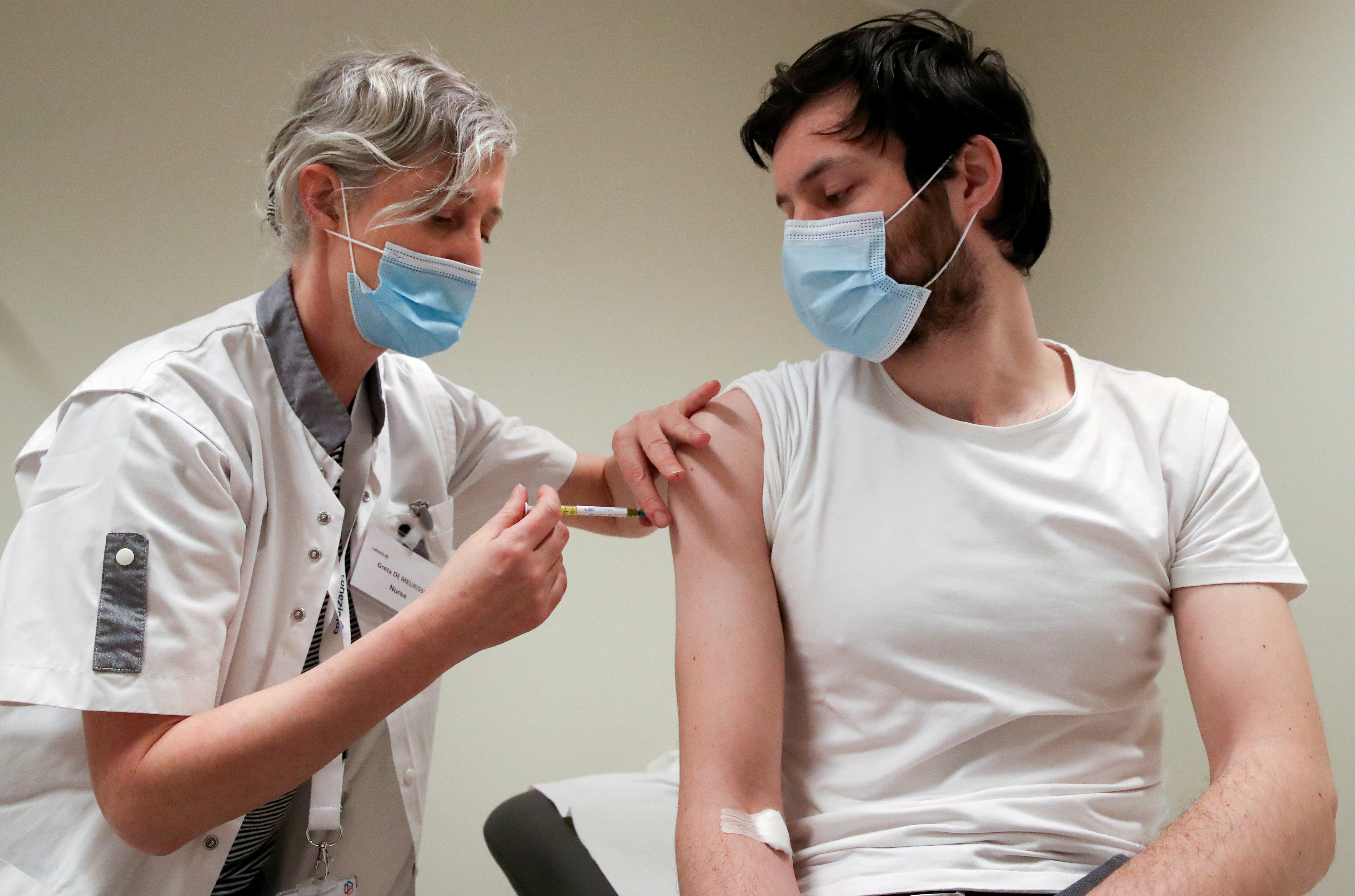
Curevac S Covid 19 Vaccine Attracts Rising Interest Reuters

Ema Starts Rolling Review Of Curevac S Covid 19 Vaccine Pink Sheet

Curevac Drops Covid 19 Vaccine Candidate Puts Focus On 2nd Generation Mrna Program
Curevac Says Its Covid 19 Vaccine Can Be Stored At Standard Refrigerator Temperature Pmlive

Coronavirus Germany S Curevac Vaccine Only 47 Effective News Dw 16 06 2021

Curevac To Trial Covid 19 Vaccine In Germany And Belgium

Curevac S Covid 19 Vaccine Candidate Only 47 Effective

Curevac S Credibility Unravels Along With Its Covid 19 Vaccine Evaluate